While there is no standardized risk value of pancreatitis associated with GLP-1 receptor agonists, as the risk would likely vary from patient to patient, pooled data consistently suggest the risk of pancreatitis is not significantly increased compared to placebo.
A recently published 2025 meta-analysis included 11 trials involving 85,373 participants to evaluate the effects of glucagon-like peptide-1 receptor agonists (GLP-1RAs). The analysis found the risk of acute pancreatitis to not be significantly increased with the use of GLP-1RAs compared to placebo (hazard ratio [HR] 1.02; 95% confidence interval [CI] 0.78 to 1.33). Overall, there was no difference in the risk of serious adverse events, including acute pancreatitis and severe hypoglycemia, between GLP-1RAs and placebo (risk ratio [RR] 0.95; 95% CI 0.90 to 1.01). However, it should be noted that treatment discontinuation due to adverse events occurred more frequently with GLP-1RAs compared to placebo (RR 1.51; 95% CI 1.18 to 1.94). [1]
Another meta-analysis published in 2024 evaluated the safety and efficacy of GLP-1RAs on cardiovascular events in overweight or obese non-diabetic patients. A total of 10 randomized controlled trials, comprising 29,325 patients, were included for ana...
READ MORE→
A search of the published medical literature revealed
1 study investigating the researchable question:
What is the frequency of pancreatitis with semaglutide or tirzepatide?
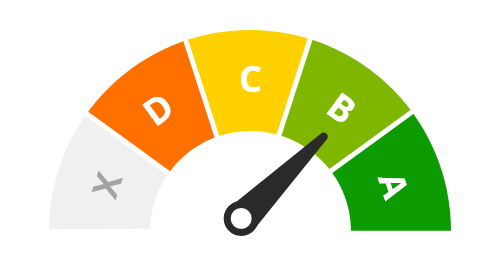
Level of evidence
B - One high-quality study or multiple studies with limitations
READ MORE→
[1] Badve SV, Bilal A, Lee MMY, et al. Effects of GLP-1 receptor agonists on kidney and cardiovascular disease outcomes: a meta-analysis of randomised controlled trials. Lancet Diabetes Endocrinol. 2025;13(1):15-28. doi:10.1016/S2213-8587(24)00271-7
[2] Singh S, Garg A, Tantry US, Bliden K, Gurbel PA, Gulati M. Safety and efficacy of glucagon-like peptide-1 receptor agonists on cardiovascular events in overweight or obese non-diabetic patients. Curr Probl Cardiol. 2024;49(3):102403. doi:10.1016/j.cpcardiol.2024.102403
[3] Dankner R, Murad H, Agay N, Olmer L, Freedman LS. Glucagon-Like Pep...