Doxycycline Post Exposure Prophylaxis for Sexually Transmitted Infections
Sheena Ward, PharmD – InpharmD DI Fellow
5 min read
Doxycycline Post Exposure Prophylaxis for Sexually Transmitted Infections
Where We Are and Where We Are Going
Over 26 million sexually transmitted infections (STIs) are reported each year in the United States alone. In 2018, one in every five Americans reported having a STI. The expense of managing these illnesses in the United States amounts to around 16 billion dollars in healthcare expenditure. Chlamydia, gonorrhea, and syphilis account for $1.1 billion of those costs altogether. Half of all new infections occur in people aged 15 to 24. The following pathogens have been related to the highest incidence: Syphilis, gonorrhea, chlamydia, and trichomoniasis are all treatable, while Hepatitis B, herpes simplex virus (HSV), human immunodeficiency virus (HIV), and human papillomavirus (HPV) are all incurable.

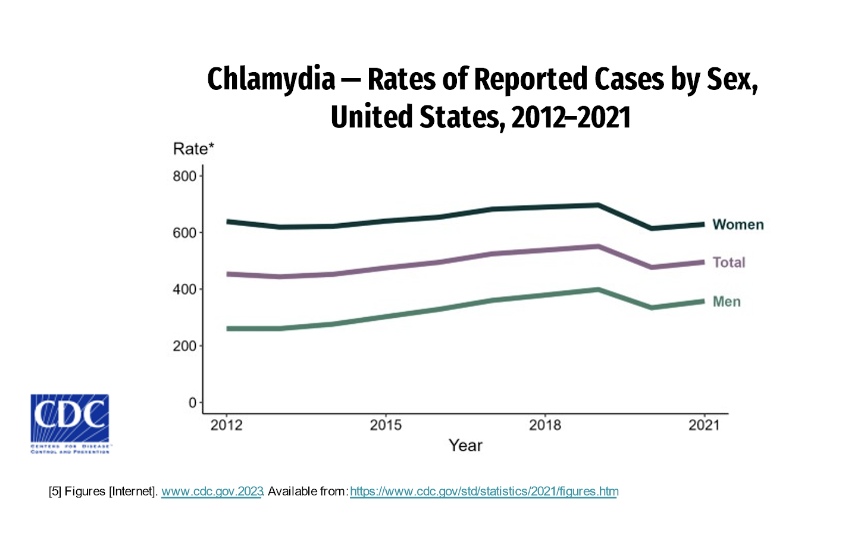
Between 2020 and 2021 the increase in chlamydia rates followed a significant decrease in rates from 2019 -2020
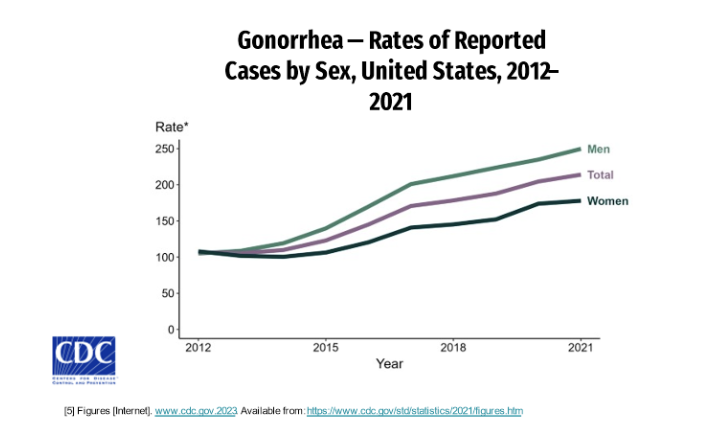
Rates of gonorrhea are consistently increasing since 2012 with higher rates in men than women
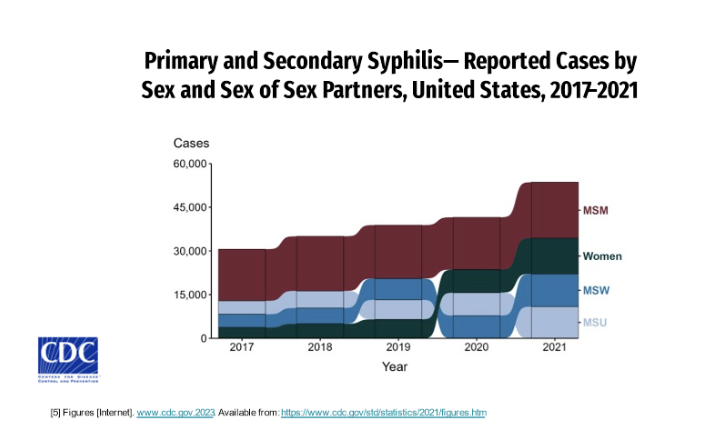
Rates of primary and secondary syphilis have increased consistently in disproportionately higher rates in men than compared to women.
The first trial to evaluate doxycycline as a prophylactic agent was conducted in France. IPERGAY was a small open-label randomized study involving 232 men who have sex with men (MSM). Patients were randomly assigned to one of two groups. A single dosage of 200 mg doxycycline was administered 24 to 72 hours following condomless intercourse in the intervention group. Those who did not take doxycycline following condomless sex were in the comparison group. Doxycycline post-exposure prophylaxis (Doxy PEP) was limited to a maximum of three times per week, which may have affected its effectiveness against gonorrhea. The primary endpoint was the first STI incidence, which was reduced in the Doxy PEP group compared to the no PEP group. A considerable reduction in chlamydia and syphilis was seen, but no reduction in gonorrhea was observed.
This information led to the second major study, Doxy PEP which was similar to the IPERGAY trial. This study was conducted in San Fransico and Seattle. The first arm consisted of doxycycline 200 mg and compared standard of care (SOC). The study was designed to look at additional outcomes of doxycycline including data on its adverse event profile, adherence rates, and antimicrobial resistance. In addition, it also evaluated the net effect of Doxy PEP. The study design was an open-label randomized trial involving both who have sex with men (MSM) and transgender women (TGW) and were living with HIV (LWHIV) or taking pre-exposure prophylaxis (Preps) and were to take Doxy PEP within 24 to 72 hours after condomless sex. Of 501 participants (327 in the preps cohort and 174 in the people living with HIV [PLWH] cohort), 67% were White, 7% Black, 11% Asian or Pacific Islander, and 30% Hispanic or Latino. In comparison to the IPERGAY trial, the doxycycline dose was not set to a three-dose per week maximum. Inclusion criteria were male sex, at least 18 years of age, LWHIV or on PREP who had a history of condomless anal or oral sex w/men and had received a diagnosis of gonorrhea, chlamydia, or early syphilis within the previous 12 months. The patients were randomized at a 2: 1 ratio for Doxy PEP or SOC respectively. STI testing was conducted at quarterly intervals for 12 months to test for gonorrhea, chlamydia, and syphilis and during treatment to assess for antimicrobial resistance.
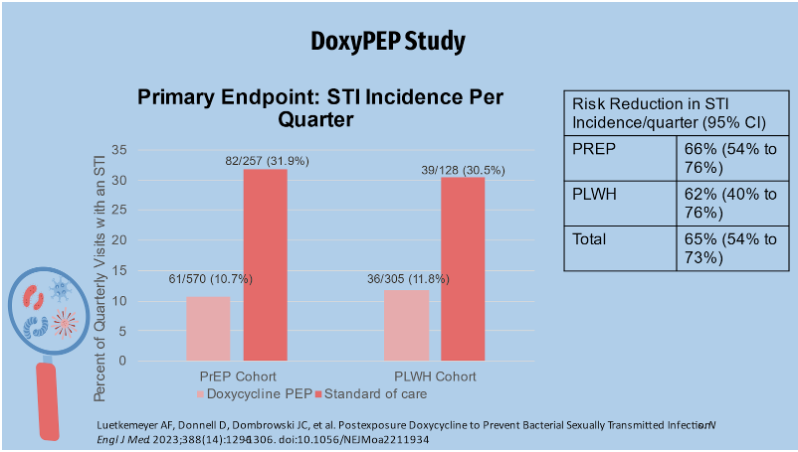
The primary endpoint of the study measured time to first STI incidence per quarter in the control arm (those receiving standard of care), and both cohorts reported slightly more than 30% of STIs per quarter. Just over 10% of STIs were reported in the Doxy PEP arm. Overall, doxycycline reduced the incidence of the three STIs by about two-thirds when compared to SOC. This graph displays data organized by individual STIs.
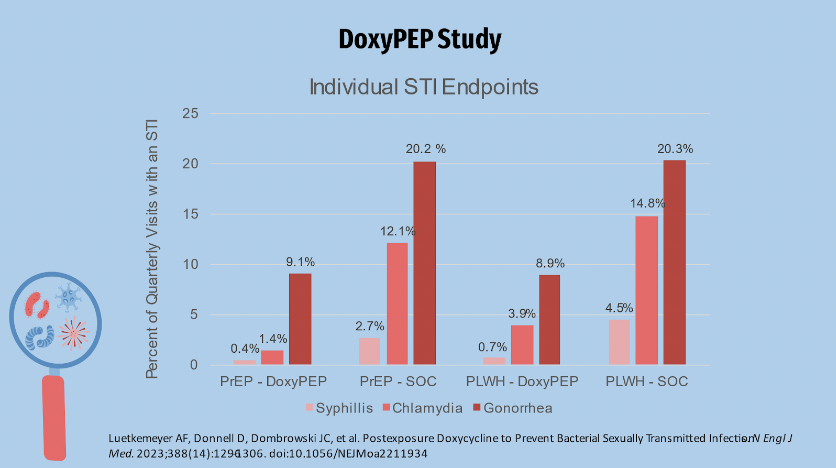
Gonorrhea was the most common of the individual STI endpoints. The Doxy PEP study showed a reduction in the incidence of gonorrhea in the Doxy PEP arm in terms of efficacy for gonorrhea in both arms. This was in contrast to the IPERGAY trial, which found no difference in the incidence of gonorrhea between Doxy PEP and no PEP. Possible explanations will be provided later. Chlamydia was the second most common endpoint, with syphilis being the least common. The quarterly incidence rates of chlamydia and syphilis are similar to those of gonorrhea. At least one STI was diagnosed in the prep cohort in 67 of 571 (11.7%) quarterly visits in the doxycycline group and 82 of 257 (31.0%) patients in the SOC group. This equates to an absolute difference of approximately 21.2% and a relative risk of approximately 0.34 in favor of doxycycline over SOC. In the LWHIV cohort, STI was diagnosed in approximately 36/305 (11.8%) of quarterly visits in the doxy group and 39/128 (30.5% of visits in the soc group, for an absolute difference of 18 percentage points and a relative risk of 0.38 favoring doxycycline over SOC in STI reduction. Looking at the graph, the darker blue shading represents high-level resistance while the lighter shading represents low-level resistance.
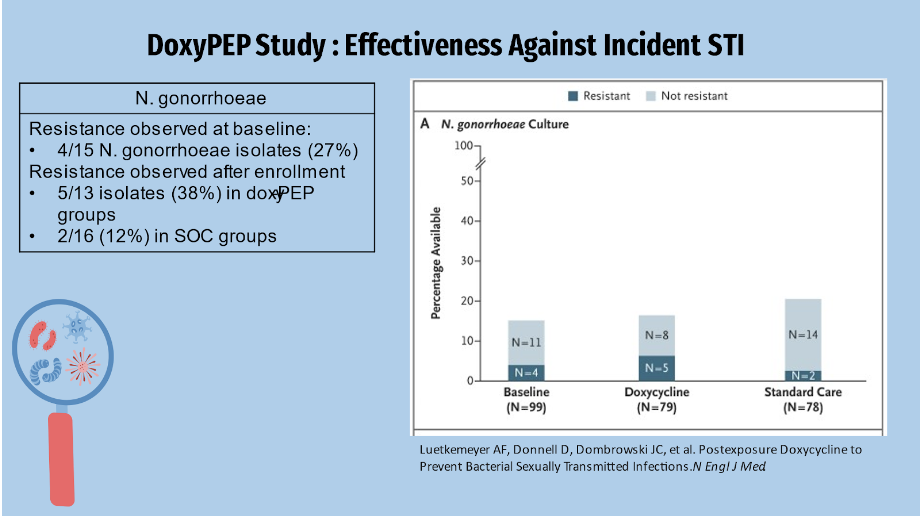
Tetracycline resistance was found in 4 of 15 Neisseria Gonorrhea isolates, or about 27% of participants with gonorrhea at baseline. Resistance was observed in 5 of 13 isolates after enrollment, accounting for approximately 38% in the Doxy PEP group and 12% in the SOC group. The higher gonorrhea resistance pattern may explain why doxycycline is not used as a first-line treatment for gonorrhea.
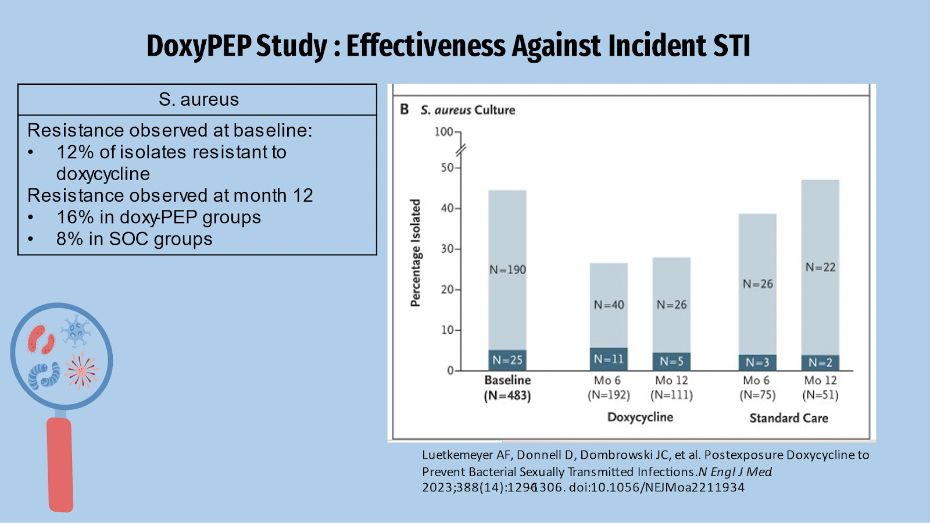
Doxycycline resistance was found in 12% of S. aureus isolates at the start of the study. After enrollment, we see that the resistance pattern in the Doxy PEP group remained higher than in the SOC group by 16% and 8%, respectively. Because doxycycline is used to treat MRSA skin and soft tissue infections, this research could have clinical implications.
At enrollment, the median number of sexual partners was 9, and the median number of sexual acts per month was 5, with 90% of these acts being condomless. At the follow-up, there was no significant difference in adherence in the Doxy PEP arm. Adherence was reported to be significantly high at the end of the study, with 86% of patients in the doxy pep arm reporting Doxy-PEP use as always/often within 72 hours of condomless anal or vaginal sex. In terms of strict adherence, 71% of Doxy PEP patients reported never missing a dose of doxycycline after condomless sex. The average amount of doxycycline given per month was four doses. A laboratory abnormality caused by elevated liver enzymes occurred in one case. There were five reports of adverse events, three of which were diarrheal and two of which were headaches or migraines. In the Doxy PEP arm, 89% of patients said it was acceptable or very acceptable. This information could have an impact on the safety and acceptability of doxycycline. Doxy-PEP given within 72 hours of condomless sex reduces incident gonorrhea, chlamydia, and early syphilis by two-thirds in MSM and TGW who have had a bacterial STI in the previous year. Doxy-PEP significantly reduced the incidence of all STIs, including gonorrhea, in PrEP and PLWH cohorts. In comparison to the IPERGAY trial, doxycycline reduced incident gonorrhea by approximately 55%. It is hypothesized that increased doxycycline exposure improved gonorrhea effectiveness. The effectiveness of doxy-PEP against Chlamydia was 88% in the PrEP cohort and 74% in the PLWH cohort. Although the number of syphilis diagnoses was low, the Doxy-PEP group had a lower incidence than the SOC group. Potential implications for differences include increased doxycycline exposure and improved adherence. Higher doxycycline exposure may have increased its effectiveness against gonorrhea. Chlamydia doxy pep treatment was 88% effective in the Doxy PEP group and 74% effective in the people LWHIV cohort. Difficulties in determining condomless intercourse and event-driven PEP use hampered measuring adherence to doxy-PEP. Quarterly computer-assisted surveys were used to collect information on sexual activity and doxycycline use, but recall was limited. Tetracycline susceptibility findings were available in only 17% of gonorrhea end points because only half of the subjects with incident gonorrhea infections during follow-up had N. gonorrhoeae culture acquired before treatment, and because extragenital infections have reduced cultivability. The availability of N. gonorrhoeae culture was comparable in the IPERGAY investigation. The Doxy PEP study discovered 27% baseline tetracycline resistance, which corresponds to the 19.7% resistance discovered in the 2020 U.S. Gonococcal Isolate Surveillance Project data. TSGW enrolled at a rate of less than 5%, limiting the sample's generalizability. Acceptability, adherence, and STI rates may vary depending on the environment; this study was conducted in two West Coast cities. In conclusion, doxycycline protected against all bacterial STIs, including gonorrhea. It was reported as safe and well tolerated, had high adherence and acceptability and is generally inexpensive.
The GOGO Vax study is currently in phase 3 and is evaluating the efficacy of 4CMenB (Bexsero) in preventing GC infection. At 0 and 3 months, subjects received two doses of Bexsero or two doses of placebo (0.5 mL NS). All subjects will be tracked for 24 months. Participants enrolled from 11/2021 to 4/2023. The results will be available in 2025. The ANRS DoxyVAC study was influenced by the GOGO Vax study. Doxycycline and the vaccine did not interact, according to preliminary findings. At a median follow-up of 9 months, 49 subjects were infected, with 36 of them receiving no PEP and 13 receiving Doxy PEP. The hazard ratio was 0.16 (95% CI 0.08 to 0.30, p 0.0001).
Doxycycline post-exposure prophylaxis (doxycycline PEP) has been shown to reduce chlamydia, gonorrhea, and syphilis infections and represents a new approach to addressing STI prevention in populations at high risk for these infections, according to the CDC's proposed guidelines for bacterial STI prevention. Doxycycline PEP, when available, should be used in conjunction with a comprehensive sexual health approach that includes risk reduction counseling, STI screening and treatment, approved vaccination, and connection to HIV pre-exposure prophylaxis (PrEP), HIV care, or other services, as needed. The proposed guidelines are intended to provide healthcare practitioners with up-to-date clinical guidance on the use of doxycycline PEP for preventing bacterial STI infections.
References:
[1] Luetkemeyer AF, Donnell D, Dombrowski JC, et al. Postexposure Doxycycline to Prevent Bacterial Sexually Transmitted Infections. N Engl J Med. 2023;388(14):1296-1306. doi:10.1056/NEJMoa2211934
[2] Molina JM, Charreau I, Chidiac C, et al. Post-exposure prophylaxis with doxycycline to prevent sexually transmitted infections in men who have sex with men: an open-label randomised substudy of the ANRS IPERGAY trial. Lancet Infect Dis. 2018;18(3):308-317. doi:10.1016/S1473-3099(17)30725-9
[3] Incidence, prevalence, and cost of sexually transmitted infections in the united states | fact sheets | newsroom | nchhstp | cdc.
[4] Sexually transmitted infections (Stis). Available from: https://www.who.int/health-topics/sexually-transmitted-infections#tab=tab_1
[5] Figures [Internet]. www.cdc.gov.2023. Available from: https://www.cdc.gov/std/statistics/2021/figures.htm
[6] Home page for MMWR. Centers for Disease Control and Prevention. September 28, 2023. Accessed October 5, 2023. https://www.cdc.gov/mmwr/index.html.
[7] Molina J-M et al. ANRS 174 DOXYVAC: an open-label randomized trial to prevent STIs in MSM on PrEP. Conference on Retroviruses and Opportunistic Infections, Seattle, abstract 119, 2023.
[8] UPDATE: Sexually transmitted infections - piedmont healthcare. Accessed September 29, 2023. https://www.piedmont.org/media/file/CME-PCP-STI-Update.pdf.
Share