Due to the absence of supportive data and heterogeneity of specific probiotic products available, none are particularly recommended for treating or preventing C. difficile. The 2021 American College of Gastroenterology (ACG) guidelines recommend against probiotics for the prevention of Clostridioides difficile infection (CDI) in patients being treated with antibiotics (primary prevention) and for the prevention of CDI recurrence (secondary prevention). While the 2021 Infectious Diseases Socie...
In the setting of Clostridioides difficile infection (CDI), the 2021 American College of Gastroenterology (ACG) guidelines recommend against probiotics for the prevention of CDI in patients being treated with antibiotics (primary prevention) and for the prevention of CDI recurrence (secondary prevention). Since probiotics are marketed as dietary supplements without strict oversight by the U.S. Food and Drug Administration required for drugs, the evidence for the use of probiotics is generally limited. Hence, manufacturers have little incentive to conduct clinical trials to support specific indications. Additionally, data based on case reports revealed several risks associated with the use of probiotics including bloodstream infections in critically ill patients. Microbiome analyses have also observed probiotics to impede normal recolonization of the colon after antibiotic courses. Evidence to support probiotics for CDI comes primarily from meta-analyses that pooled data from small t...
READ MORE→
A search of the published medical literature revealed
2 studies investigating the researchable question:
Is there any probiotic that is clinically relevant in treating or preventing C. Diff?
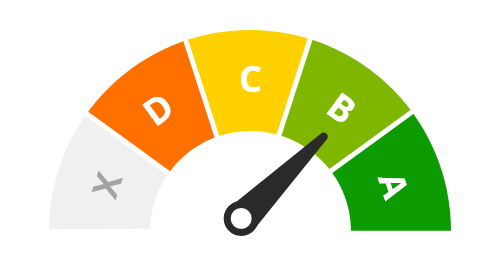
Level of evidence
B - One high-quality study or multiple studies with limitations
READ MORE→
[1] Kelly CR, Fischer M, Allegretti JR, et al. ACG Clinical Guidelines: Prevention, Diagnosis, and Treatment of Clostridioides difficile Infections [published correction appears in Am J Gastroenterol. 2022 Feb 1;117(2):358]. Am J Gastroenterol. 2021;116(6):1124-1147. doi:10.14309/ajg.0000000000001278
[2] Allen SJ, Wareham K, Wang D, et al. Lactobacilli and bifidobacteria in the prevention of antibiotic-associated diarrhoea and Clostridium difficile diarrhoea in older inpatients (PLACIDE): a randomized, double-blind, placebo-controlled, multicentre trial. Lancet. 2013;382(9900):1249-1257. doi:10.1016/S0140-6736(13)61218-0
[3] Goldenberg JZ, Yap C, Lytvyn L, et al. Probiotics for the prevention of Clostridium difficile-associated diarrhea in adults and children. Cochrane Database Syst Rev. 2017;12(12):CD006095. Published 2017 Dec 19. doi:10.1002/14651858.CD006095.pub4
[4] McFarland LV, Ship N, Auclair J, Millette M. Primary prevention of Clostridium difficile infections with a specific probiotic combining Lactobacillus acidophilus, L. casei, and L. rhamnosus strains: assessing the evidence. J Hosp Infect. 2018;99(4):443-452. doi:10.1016/j.jhin.2018.04.017
[5] Gao XW, Mubasher M, Fang CY, Reifer C, Miller LE. Dose-response efficacy of a proprietary probiotic formula of Lactobacillus acidophilus CL1285 and Lactobacillus casei LBC80R for antibiotic-associated diarrhea and Clostridium difficile-associated diarrhea prophylaxis in adult patients. Am J Gastroenterol. 2010;105(7):1636-1641. doi:10.1038/ajg.2010.11
[6] Shen NT, Maw A, Tmanova LL, et al. Timely Use of Probiotics in Hospitalized Adults Prevents Clostridium difficile Infection: A Systematic Review With Meta-Regression Analysis. Gastroenterology. 2017;152(8):1889-1900.e9. doi:10.1053/j.gastro.2017.02.003
[7] Esmaeilinezhad Z, Ghosh NR, Walsh CM, et al. Probiotics for the prevention of Clostridioides difficile-associated diarrhea in adults and children. Cochrane Database Syst Rev. 2025;9(9):CD006095. Published 2025 Sep 11. doi:10.1002/14651858.CD006095.pub5
[8] Barker AK, Duster M, Valentine S, et al. A randomized controlled trial of probiotics for Clostridium difficile infection in adults (PICO). J Antimicrob Chemother. 2017;72(11):3177-3180. doi:10.1093/jac/dkx254
[9] Ma Y, Yang JY, Peng X, Xiao KY, Xu Q, Wang C. Which probiotic has the best effect on preventing Clostridium difficile-associated diarrhea? A systematic review and network meta-analysis. J Dig Dis. 2020;21(2):69-80. doi:10.1111/1751-2980.12839
[10] Li Z, Zhu G, Li C, Lai H, Liu X, Zhang L. Which Probiotic Is the Most Effective for Treating Acute Diarrhea in Children? A Bayesian Network Meta-Analysis of Randomized Controlled Trials. Nutrients. 2021;13(12):4319. Published 2021 Nov 29. doi:10.3390/nu13124319
[11] Johnson S, Lavergne V, Skinner AM, et al. Clinical Practice Guideline by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA): 2021 Focused Update Guidelines on Management of Clostridioides difficile Infection in Adults. Clin Infect Dis. 2021;73(5):e1029-e1044. doi:10.1093/cid/ciab549
[12] McDonald LC, Gerding DN, Johnson S, et al. Clinical Practice Guidelines for Clostridium difficile Infection in Adults and Children: 2017 Update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clin Infect Dis. 2018;66(7):e1-e48. doi:10.1093/cid/cix1085
[13] Su GL, Ko CW, Bercik P, et al. AGA Clinical Practice Guidelines on the Role of Probiotics in the Management of Gastrointestinal Disorders. Gastroenterology. 2020;159(2):697-705. doi:10.1053/j.gastro.2020.05.059
[14] Guarner F, Sanders ME, Szajewska H, et al. World Gastroenterology Organisation Global Guidelines: probiotics and prebiotics. Published February 2023. Accessed March 3, 2026. https://www.worldgastroenterology.org/UserFiles/file/guidelines/probiotics-and-prebiotics-english-2023.pdf