Current guidelines (GOLD 2025 and GINA 2025) recommend systemic corticosteroids for acute exacerbations but do not address their routine concomitant use with inhaled corticosteroids (ICS). Evidence from randomized controlled trials and systematic reviews in acute asthma demonstrates insufficient or inconsistent clinical benefit with the addition of ICS to systemic corticosteroids, although one meta-analysis reported reduced hospital admissions with high-dose ICS plus systemic corticosteroids ...
Both the 2025 GOLD and GINA guidelines for COPD and asthma, respectively, do not address concomitant use of inhaled corticosteroids (ICS) and systemic corticosteroids. While in the GOLD guidelines, exacerbations of COPD may require treatment using systemic corticosteroids that could overlap with current ICS, this is an acute phase of treatment and not meant for routine exposure. GOLD does not recommend systemic corticosteroids for more than 5 days, which includes hospital exposure. The same principle applies within the GINA guidelines which recommends systemic corticosteroids primarily for moderate or severe exacerbations, especially if patients fail to respond to ICS-containing medications. However, GINA does provide slightly more context, suggesting systemic corticosteroids can be continued for 5-7 days in adults, or 3-5 days in children post-exacerbation. During this time, maintenance ICS-containing medication dose may be increased in the next 2-4 weeks, meaning that concomitant ...
READ MORE→
A search of the published medical literature revealed
2 studies investigating the researchable question:
Is there an indication for using concomitant Inhaled and systemic corticosteroids routinely?
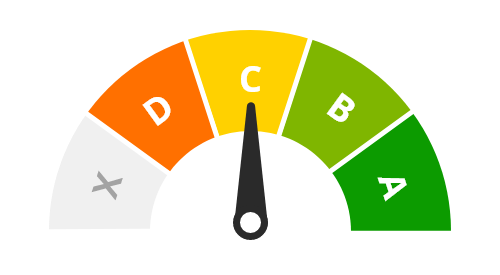
Level of evidence
C - Multiple studies with limitations or conflicting results
READ MORE→
[1] 2025 GOLD Report. Global initiative for chronic obstructive lung disease. Accessed February 25, 2026. https://goldcopd.org/2025-gold-report/
[2] Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention, 2025 update. Accessed February 25, 2026. https://ginasthma.org
[3] Edmonds ML, Milan SJ, Camargo CA Jr, Pollack CV, Rowe BH. Early use of inhaled corticosteroids in the emergency department treatment of acute asthma. Cochrane Database Syst Rev. 2012;12(12):CD002308. Published 2012 Dec 12. doi:10.1002/14651858.CD002308.pub2
[4] Edmonds ML, Milan SJ, Brenner BE, Camargo CA Jr, Rowe BH. Inhaled steroids for acute asthma following emergency department discharge. Cochrane Database Syst Rev. 2012;12(12):CD002316. Published 2012 Dec 12. doi:10.1002/14651858.CD002316.pub2
[5] Kearns N, Maijers I, Harper J, Beasley R, Weatherall M. Inhaled Corticosteroids in Acute Asthma: A Systemic Review and Meta-Analysis. J Allergy Clin Immunol Pract. 2020;8(2):605-617.e6. doi:10.1016/j.jaip.2019.08.051